A) aromatic
B) antiaromatic
C) nonaromatic
E) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are aromatic? 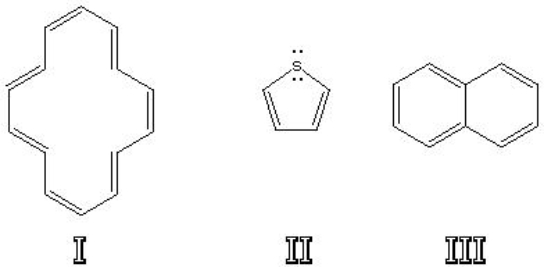
A) I
B) II
C) III
D) I and II
E) II and III
F) I and III
G) none of the above
H) all of the above
J) B) and G)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are aromatic?
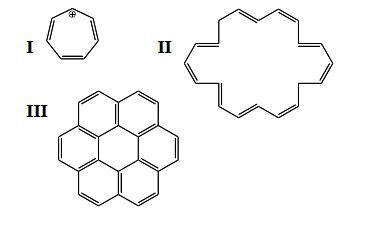
A) I
B) II
C) III
D) I and II
E) II and III
F) I and III
G) none of the above
H) all of the above
J) G) and H)
Correct Answer

verified
F
Correct Answer
verified
Multiple Choice
To be aromatic, a compound must be ___ , ___ , contain alternating double and single bonds and have A(n) ___ of electrons.
A) noncyclic, coplanar, even number
B) cyclic, symmetric, odd number
C) cyclic, planar, Huckel
D) cyclic, aliphatic, Huckel
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct name for the following? 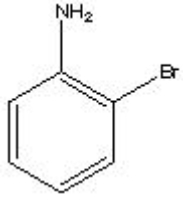
A) o-bromoaniline
B) 2-bromoaniline
C) 1-amino-2-bromobenzene
D) all of the above
F) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct name for the following? 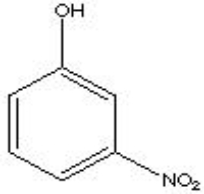
A) o-nitrophenone
B) p-hydroxynitrobenzene
C) 3-nitroacetophenone
D) m-nitrophenol
F) A) and B)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A new monocyclic ring is formed. The pi-electron energy of the ring is calculated and found to be 25 kJ/mole lower than the pi-electron energy of the corresponding open chain form of the ring with the same number of carbons and double bonds.
A) aromatic
B) antiaromatic
C) nonaromatic
E) A) and B)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following represents the two major fragments that are seen when monoalkylbenzenes fragment in a Mass Spectrometer? 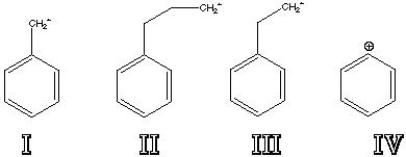
A) I and II
B) I and III
C) I and IV
D) II and III
E) III and IV
F) II and IV
H) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In NMR, aromatic hydogens resonate at ___ ppm while carbons resonate at ___ ppm. In IR spectroscopy, aromatic hydrogens absorb at ___ cm-1.
A) 3-4, 100-170, 1450-1600
B) 6-9.5, 100-170, 3030
C) 3-4, 100-170, 2250
D) 6-9.5, 100-170, 1450-1600
F) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
___ is the general name for all monocyclic structures that contain alternating double and single bonds.
A) Annulenes
B) Cumulenes
C) Aromatics
D) Antiaromatics
F) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 10 of 10
Related Exams